 |
JAPAN APPROVALS DATABASE (JAD) |
| Jouhou Koukai Publishing, a Jouhou Koukai business |
ISSN 1550-3399 |
|
|
 |
JAPAN APPROVALS DATABASE (JAD) |
| Jouhou Koukai Publishing, a Jouhou Koukai business |
ISSN 1550-3399 |
|
|
or |
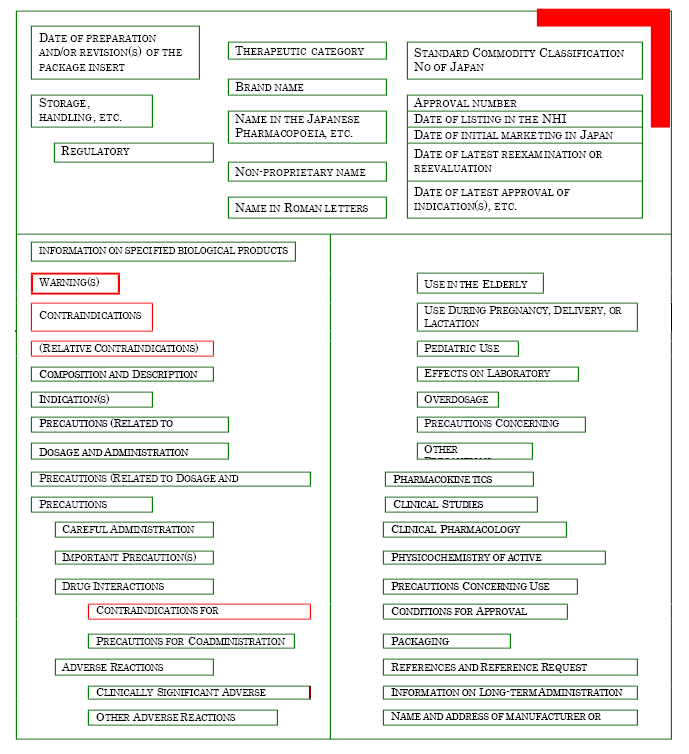
The layout of the PI is uniform and as shown on the illustration below with red color coding designed to attract the attention to the most critical information. Shown below are:
 |
Reports: |
The full text in English of the Japanese Package Insert for Mobic (meloxicam) registered to and marketed in Japan by Nippon Boehringer Ingelheim KK. Formatted, indexed and hyperlinked. 34-page Drugs Lifecycle document. |
Source: JPMA