HEPATITIS C RELIEF ON THE WAY
Tokyo - Plaintiffs of one of the most
publicized challenges to the drug approval practice of the Japanese Government finally received the coveted
apology delivered in person by the PM Yasuo Fukuda. Moreover, what plaintiffs
specifically insisted is the new law to explcitely point out the responsibility of the state for this mass tragedy. Indeed, the preamble of the bill states, "The government should admit its responsibility for causing huge harm to the victims of the infection and for failing to prevent the harm from spreading, and offer a heartfelt apology to them."
Plaintiffs of one of the most
publicized challenges to the drug approval practice of the Japanese Government finally received the coveted
apology delivered in person by the PM Yasuo Fukuda. Moreover, what plaintiffs
specifically insisted is the new law to explcitely point out the responsibility of the state for this mass tragedy. Indeed, the preamble of the bill states, "The government should admit its responsibility for causing huge harm to the victims of the infection and for failing to prevent the harm from spreading, and offer a heartfelt apology to them."
As reported in the mass media, the House of Representatives passed a bill Tuesday to offer blanket relief to people with hepatitis C caused by tainted blood products, paving the way for its passage through the opposition-controlled House of Councilors for enactment into law on Friday. An upper house committee on health, welfare, and labor agreed during an informal meeting of executive members to hold a plenary session vote at the upper house after holding a vote at a committee meeting on Thursday. The ruling and opposition parties have both given their approval to the bill. Prime Minister Yasuo Fukuda told reporters the same day, "I really want the bill to be passed. The bill was first introduced to the lower house by the ruling coalition of the Liberal Democratic Party and the New Komeito party on Monday. But the coalition retracted it to pave the way for both ruling and opposition lawmakers to jointly present an almost identical version of the bill to the plenary session in the form of legislation proposed by the committee chairman. Under the relief bill, people who contracted hepatitis C after being administered with contaminated blood products, such as fibrinogen, will receive compensation ranging from 12 million yen to 40 million yen per person depending on the severity of their suffering. The government and drug makers will pay the relief by setting up a fund at the Pharmaceuticals and Medical Devices Agency. The bill was crafted by the ruling parties following Fukuda's instruction in late December. The bill's contents were agreed with hepatitis C sufferers who have filed damages suits against the state and drug makers. In a related move, the plaintiffs have decided to conclude next week a basic agreement with the state on an amicable settlement and enter procedures for out-of-court settlements. The lawsuits were filed with five district courts across Japan starting in October 2002 by people who contracted hepatitis C after having been administered the products to stop bleeding during operations or the delivery of babies from around 1970 to the early 1990s. The combined number of plaintiffs currently stands at around 200.
The passing of the bill has been promoted politically as badly needed beneficial publicity for the ruling coalition, although its "blanket" nature was openly ridiculed in the press:
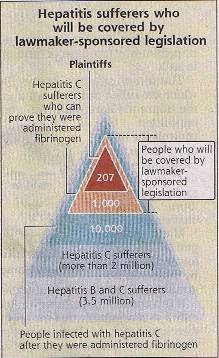 "Even though it has been dubbed "blanket relief," the bill for people infected with the hepatitis C virus via tainted blood products is believed to cover only 1,000 people--a very small proportion of the 3.5 million people (see diagram at the left) suffering either from hepatitis B or hepatitis C, as a result of drug-induced infection. The bill aims to provide relief to about 200 hepatitis C sufferers who filed damages suits against the state and drugmakers. That is why the bill only focuses on fibrinogen and a limited number of other blood products referred to by plaintiffs as sources of infection. About 800 people who do not number among the plaintiffs have the medical records to prove they contracted the virus after being administered the same kinds of blood products mentioned by the plaintiffs. Therefore, about 1,000 people will be covered by the bill. The average payment per person will be about 20 million yen, so the state and drugmakers are expected to pay out about 20 billion Yen in total. If the bill had included a wider range of people for coverage--say several tens or hundreds of thousands--the total payment might have ended up in the trillions of yen. The government and ruling parties clearly restricted the range of those eligible for relief after considering how to limit the state's financial burden."
"Even though it has been dubbed "blanket relief," the bill for people infected with the hepatitis C virus via tainted blood products is believed to cover only 1,000 people--a very small proportion of the 3.5 million people (see diagram at the left) suffering either from hepatitis B or hepatitis C, as a result of drug-induced infection. The bill aims to provide relief to about 200 hepatitis C sufferers who filed damages suits against the state and drugmakers. That is why the bill only focuses on fibrinogen and a limited number of other blood products referred to by plaintiffs as sources of infection. About 800 people who do not number among the plaintiffs have the medical records to prove they contracted the virus after being administered the same kinds of blood products mentioned by the plaintiffs. Therefore, about 1,000 people will be covered by the bill. The average payment per person will be about 20 million yen, so the state and drugmakers are expected to pay out about 20 billion Yen in total. If the bill had included a wider range of people for coverage--say several tens or hundreds of thousands--the total payment might have ended up in the trillions of yen. The government and ruling parties clearly restricted the range of those eligible for relief after considering how to limit the state's financial burden." The details are still emerging, but under the bill, people who were infected with hepatitis C after being administered with virus-contaminated blood products, including fibrinogen
 , that were produced on certain dates between 1964 and 1987 will receive up to 40 million Yen per person, depending on the severity of their condition. The following compensation schedule is planned: Those who contracted hepatitis C through the blood products and whose conditions progressed to cirrhosis or liver cancer will receive 40 million Yen, as will relatives of people who have died. Those with chronic hepatitis C will each receive 20 million Yen, and hepatitis C positive patients will get 12 million Yen. A fund to pay the compensation, whose initial cost is estimated at 20.5 billion Yen, will be established by the government and drugmakers once the bill is passed.
, that were produced on certain dates between 1964 and 1987 will receive up to 40 million Yen per person, depending on the severity of their condition. The following compensation schedule is planned: Those who contracted hepatitis C through the blood products and whose conditions progressed to cirrhosis or liver cancer will receive 40 million Yen, as will relatives of people who have died. Those with chronic hepatitis C will each receive 20 million Yen, and hepatitis C positive patients will get 12 million Yen. A fund to pay the compensation, whose initial cost is estimated at 20.5 billion Yen, will be established by the government and drugmakers once the bill is passed.
Importantly, the relief bill also orders the government to reveal the names of the hospitals that administered the tainted blood products and encourage people who may have gotten hepatitis C through the blood products to undergo checkups. Those who have credible documents must file lawsuits and submit a court decision that acknowledges they are victims who are entitled to compensation as well.
However, even now a doubt is expressed on how the new law shall be implemented aside from the 200 clear-cut plaintiffs and the 800 with legitimate records. No documentation has been retained in many of the hospitals, other establishments have been closed, and individual patients have lost or destroyed their own copies. It is projected that just a fraction of the estimated 9,000 other patients may received any relief.
In Japan the treatment of HCV related disorders is well established and insured. On December 12, 2007 the Central Social Insurance Medical Council decided on the reimbursable cost for Peginterferon / Ribavirin treatment of patients with chronic hepatitis C for daily and weekly regimens.
For hepatitis and interferon information in Japan see also related readings and reports.
Data sourced from Kyodo, YS; additional content by JKS staff
