September 2007
StatisticsThe Master Files (MF) registration system was launched in Japan in 2005 under the provisions of the amended Pharmaceutical Affairs Law, which took effect on April 1, 2005. The following statistics summarize the status of MF as of September 2007:
the registration of MF started on May 18, 2005 and latest entries of August 2007
the total number of registered MF is exceeding 3,000
38 of the registered MF are now delisted (withdrawn or revoked)
in 2005 a total of 1,327 MF were registered and up to date 966 MF are registered in 2006
the current over 3,000 MF are registered by more than 700 registrants
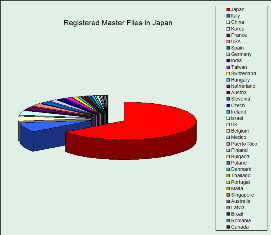
the current 3,000 MF are registered by registrants from 50 countries, as the top 5 country (in descending order) are Japan, Italy, China, Korea, and USA
the registrants from Japan are nearly 50% and they have registered 65% of all MF
in the four permitted categories for registration, out of the registered 3,000 MF, about 30 MF are registered as "Miscellaneous", 1 as "Additive", and the rest are registered as "Pharmaceutical ingredients". Few registration has been made for the fourth permitted category - "Ingredients for medical devices"
677 MF are registered for products compliant to the standards of the Japanese Pharmacopeia (JP) and 172 MF are registered for products complaint to the Non-JP drugs standards
the top 3 registrants from Japan are Alps Yakuhin, Ajinomoto and Japan Red Cross
 Further and more complete statistical details from Japan Master Files Database can be extracted by subscribers by using the built-in "Advanced Search" function in any of the 12 search categories.
Further and more complete statistical details from Japan Master Files Database can be extracted by subscribers by using the built-in "Advanced Search" function in any of the 12 search categories.
The graph below represents a snapshot of the JAD-MF (taken by November 2006) and provided here for illustrative purpose only. Subscribers to JAD-MF have access to up-to date graph and oter analytical tools.
Additional inquiries to Regulatory Department by email
All materials on this web site are protected by copyrights. Jouhou Koukai and Jouhou Koukai logo are service marks of JKS LLC.
Terms & Conditions | Inquiries to Regulatory Department