Approval of Medicinal Products - Overview of the process
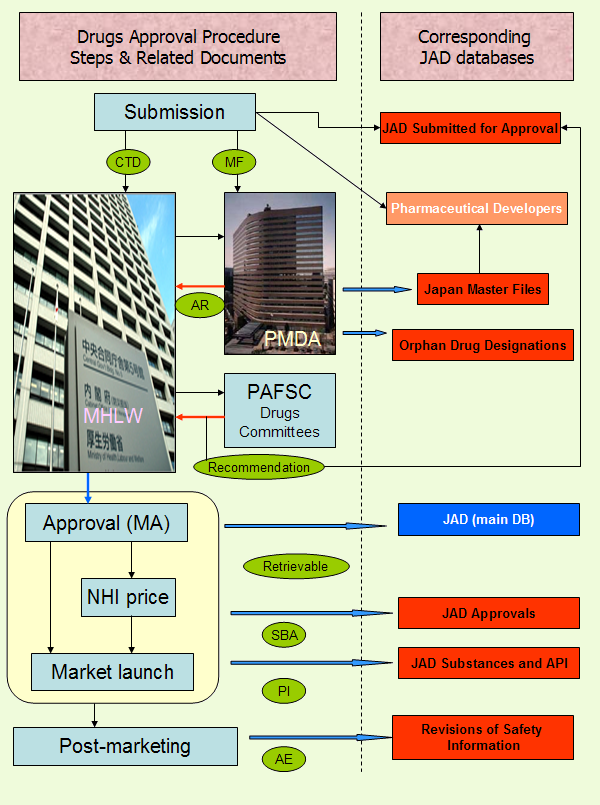
Approval process for medicinal products in Japan - and in a larger extend the part of product lifecycle from submission to post-marketing period, is largely shaped by the relations between institutions involved. The flowchart below outlines the key steps of a successful drug approval
Updated on May 7, 2007 |
Email inquiries |
Orders